Detecting early onset of clinical signs in the mouse model of Covid-19
This text was provided by Dr. Stefano Gaburro (stefano.gaburro[at]tecniplast.it), Scientific Director at Tecniplast S.p.A, Buguggiate, Italy.
Introduction
Since the start of the Covid-19 pandemic, a global effort has been made to identify the mechanisms of action of the SARS-CoV-2 (Covid19) virus and find a cure. In preclinical research, several animal models have been proposed, ranging from Syrian Hamsters up to Non-Human Primates. Unfortunately, the most widely used animal model, namely the mouse, does not properly show signs of infections because of lack of the receptor by which the virus can enter murine cells. In February 2020, Jackson Lab thawed K18-hACE2 mice, initially created for studying the SARS Virus (McCray et al., 2007), that carry the human ACE2 receptor, which is the “lock” by which the virus enters the cell with its “key” being the spike protein. This mouse model has been presented in several articles, for example:
https://www.nytimes.com/2020/03/14/science/animals-coronavirus-vaccine.html
https://www.nature.com/articles/d41586-020-00698-x
When the mice became available in the early summer of 2020, the most prominent centres in the world have started to work with them to evaluate the viral response and possible cures (drugs or vaccines). Most of the publications about infectious disease, including in vivo laboratory animal experiments, use body weight as output for animal welfare. Two recent publications indicate that body weight loss, as an indicator of clinical signs, is visible only from day 4 onwards (Jiang et al., 2020; Winkler et al., 2020). We tested the hypothesis as to whether locomotion, as a clinical sign, can anticipate subsequent body weight loss.
Methods
In collaboration with the RI-MUHC BSL3 Facility at McGill University, Montreal, we evaluated the clinical signs of these mice to SARS-CoV2 using our non-intrusive and external cage technology directly in the home rack, called the Digital Ventilated Cage (DVC®) (Iannello, 2019; Pernold et al., 2019). Eight K18-hACE2 (C57Bl/6J background) mice were exposed to the SARS-COV-2 virus, and locomotion was assessed for six days. Body weight was measured daily.
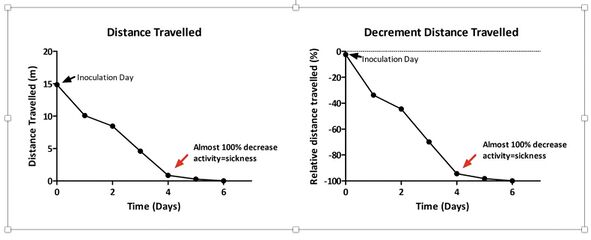
Results & Brief Discussion
The graphs show that there was a significant decrease in distance travelled of about 50% after two days, and a full decline of 98% after four days, following inoculation. Notably, after six days the animals were completely immobile. These results indicate sickness of the animal, and it is the first data of its kind showing that SARS-CoV-2 impacts in such a dramatic way on animal locomotion. In contrast, mice started to show body weight loss only after 4 days, and a decline of 15-20% 6-7 days after inoculation (data not shown). These findings are in agreement with what has been recently published (Jiang et al., 2020; Winkler et al., 2020).
It is of interest to note that locomotion results are in line with what was reported previously in other studies, namely that, after seven days of SARS inoculation, the hACE2 mouse model become very sick up and eventually died after seven days (McCray et al., 2007).
Conclusion
The home cage monitoring system demonstrated its potential to identify clinical signs of the effect of the virus. Future studies are on the way to clarify whether successful vaccine(s) can revert or at least ameliorate the SARS-CoV-2 induced clinical sign, namely hypolocomotion, in this mouse model. Since the technology can be applied in BSL-3 and 4 environments, the same system could also be used for automated, high-throughput and unbiased data collection when investigating neurological diseases such as rabies or other zoonoses.
Acknowledgments
The research was funded by MI4 at McGill University, Montreal, Canada.
References
- Iannello, F. (2019). Non-intrusive high throughput automated data collection from the home cage. Heliyon 5, e01454.
- Jiang, R.D., Liu, M.Q., Chen, Y., Shan, C., Zhou, Y.W., Shen, X.R., Li, Q., Zhang, L., Zhu, Y., Si, H.R., Wang, Q., Min, J., Wang, X., Zhang, W., Li, B., Zhang, H.J., Baric, R.S., Zhou, P., Yang, X.L., and Shi, Z.L. (2020). Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell 182, 50-58 e58.
- McCray, P.B., Jr., Pewe, L., Wohlford-Lenane, C., Hickey, M., Manzel, L., Shi, L., Netland, J., Jia, H.P., Halabi, C., Sigmund, C.D., Meyerholz, D.K., Kirby, P., Look, D.C., and Perlman, S. (2007). Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81, 813-821.
- Pernold, K., Iannello, F., Low, B.E., Rigamonti, M., Rosati, G., Scavizzi, F., Wang, J., Raspa, M., Wiles, M.V., and Ulfhake, B. (2019). Towards large scale automated cage monitoring - Diurnal rhythm and impact of interventions on in-cage activity of C57BL/6J mice recorded 24/7 with a non-disrupting capacitive-based technique. PLoS One 14, e0211063.
- Winkler, E.S., Bailey, A.L., Kafai, N.M., Nair, S., Mccune, B.T., Yu, J., Fox, J.M., Chen, R.E., Earnest, J.T., Keeler, S.P., Ritter, J.H., Kang, L.I., Dort, S., Robichaud, A., Head, R., Holtzman, M.J., and Diamond, M.S. (2020). SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol.